Delve into the captivating realm of covalent bonds with our comprehensive guide, complemented by the invaluable Student Exploration Covalent Bonds Gizmo answers. This guide empowers you to unravel the intricacies of covalent bonding, equipping you with a profound understanding of its properties, formation, and applications.
Embark on a journey of discovery as we explore the fundamental principles governing covalent bonds, examining their impact on molecular structure and behavior. Through interactive Gizmo simulations, you will gain hands-on experience in manipulating atoms and molecules, fostering a deeper comprehension of the concepts at play.
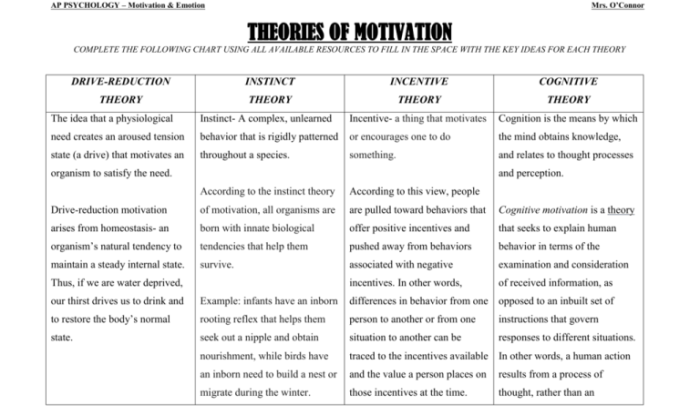
Covalent Bonds Gizmo Basics
The Covalent Bonds Gizmo is an interactive simulation that allows students to explore the properties and behavior of covalent bonds. The Gizmo features a variety of atoms and molecules that can be used to create and manipulate covalent bonds. Students can use the Gizmo to investigate the different types of covalent bonds, such as single bonds, double bonds, and triple bonds.
They can also explore the effects of different atoms and molecules on the properties of covalent bonds.
Different Types of Atoms and Molecules
The Covalent Bonds Gizmo includes a variety of atoms and molecules that can be used to create covalent bonds. These include hydrogen, carbon, nitrogen, oxygen, and chlorine. Students can use these atoms and molecules to create a wide variety of covalent compounds, such as water, carbon dioxide, and methane.
Creating and Manipulating Covalent Bonds
To create a covalent bond, students simply need to drag and drop an atom onto another atom. The Gizmo will automatically create a covalent bond between the two atoms. Students can also manipulate covalent bonds by dragging and dropping the atoms that are bonded together.
This allows students to explore the different ways that covalent bonds can be formed and broken.
Exploring Covalent Bond Properties
Bond Length
The bond length is the distance between the nuclei of the two atoms that are bonded together. The bond length is determined by the size of the atoms involved in the bond. Smaller atoms have shorter bond lengths than larger atoms.
Bond Strength
The bond strength is the amount of energy required to break a bond. The bond strength is determined by the number of bonds between the atoms and the electronegativity of the atoms involved in the bond. Bonds between atoms with large electronegativity differences are stronger than bonds between atoms with small electronegativity differences.
Bond Polarity
The bond polarity is the uneven distribution of electrons in a bond. A bond is polar if the electrons are not shared equally between the two atoms. The bond polarity is determined by the electronegativity of the atoms involved in the bond.
Bonds between atoms with large electronegativity differences are polar, while bonds between atoms with small electronegativity differences are nonpolar.
Covalent Bond Formation and Breaking
Covalent Bond Formation
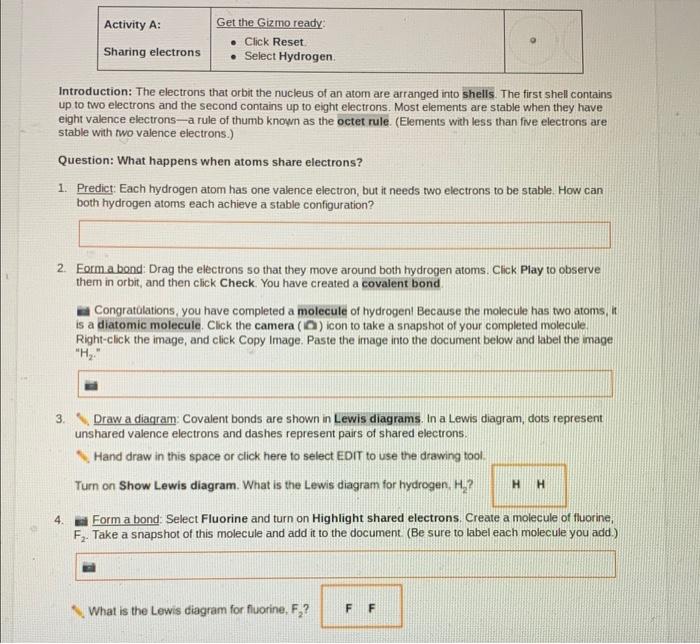
Covalent bonds are formed when two atoms share one or more pairs of electrons. The electrons are shared in order to create a stable electron configuration for both atoms. The number of electrons that are shared determines the type of covalent bond that is formed.
A single bond is formed when two atoms share one pair of electrons, a double bond is formed when two atoms share two pairs of electrons, and a triple bond is formed when two atoms share three pairs of electrons.
Covalent Bond Breaking, Student exploration covalent bonds gizmo answers
Covalent bonds can be broken when the electrons that are shared between the two atoms are no longer shared. This can happen when the atoms are heated or when they are exposed to a chemical reaction. When a covalent bond is broken, the two atoms that were bonded together become free radicals.
Applications of Covalent Bonds
Biological Molecules
Covalent bonds are essential for the formation of biological molecules, such as proteins and DNA. Proteins are made up of amino acids that are linked together by covalent bonds. DNA is made up of nucleotides that are linked together by covalent bonds.
Covalent bonds also play a role in the structure and function of other biological molecules, such as carbohydrates and lipids.
New Materials
Covalent bonds are also used in the development of new materials, such as polymers and nanomaterials. Polymers are made up of long chains of repeating units that are linked together by covalent bonds. Nanomaterials are made up of very small particles that are linked together by covalent bonds.
Covalent bonds give polymers and nanomaterials their unique properties, such as strength, durability, and lightness.
Everyday Life
Covalent bonds are used in a variety of everyday products, such as plastics and pharmaceuticals. Plastics are made up of polymers that are linked together by covalent bonds. Pharmaceuticals are made up of small molecules that are linked together by covalent bonds.
Covalent bonds also play a role in the production of other everyday products, such as food, clothing, and fuel.
FAQ Summary: Student Exploration Covalent Bonds Gizmo Answers
What is the purpose of the Covalent Bonds Gizmo?
The Covalent Bonds Gizmo is an interactive simulation tool that allows students to explore the properties and behavior of covalent bonds.
How can I use the Gizmo to create covalent bonds?
To create covalent bonds using the Gizmo, select two atoms and click on the “Bond” button. The Gizmo will automatically create a covalent bond between the two atoms.
What are the different types of covalent bonds?
There are three main types of covalent bonds: single bonds, double bonds, and triple bonds. Single bonds are formed when two atoms share one pair of electrons, double bonds are formed when two atoms share two pairs of electrons, and triple bonds are formed when two atoms share three pairs of electrons.