Embark on a comprehensive journey into the realm of acid-base theories with our meticulously crafted Acid-Base Theories Worksheet Answer Key. This invaluable resource empowers you to unravel the complexities of acid-base chemistry, providing clarity and understanding at every step.
Delving into the fundamental concepts of Arrhenius, Brønsted-Lowry, and Lewis theories, this guide illuminates the nature of acids and bases, their reactions, and their profound applications in various fields.
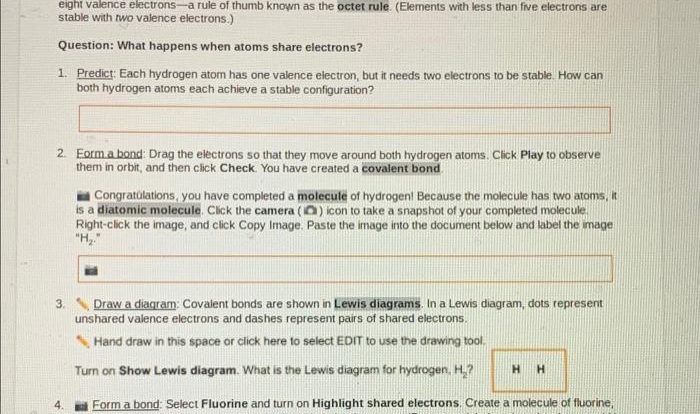
Arrhenius Theory: Acid-base Theories Worksheet Answer Key
The Arrhenius theory defines acids as substances that produce hydrogen ions (H+) when dissolved in water, while bases are substances that produce hydroxide ions (OH-) when dissolved in water. Arrhenius acids and bases are also known as ionic acids and bases, respectively.
Examples of Arrhenius acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). Examples of Arrhenius bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).
The Arrhenius theory is limited in that it only applies to aqueous solutions. It cannot explain the behavior of acids and bases in non-aqueous solvents.
Brønsted-Lowry Theory
The Brønsted-Lowry theory defines acids as substances that can donate protons (H+), while bases are substances that can accept protons. This theory is more general than the Arrhenius theory, as it applies to both aqueous and non-aqueous solutions.
Examples of Brønsted-Lowry acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH). Examples of Brønsted-Lowry bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
The Brønsted-Lowry theory is an improvement over the Arrhenius theory because it can explain the behavior of acids and bases in both aqueous and non-aqueous solvents.
Lewis Theory
The Lewis theory defines acids as substances that can accept an electron pair, while bases are substances that can donate an electron pair. This theory is the most general of the three theories, as it applies to both aqueous and non-aqueous solutions and can explain the behavior of a wider range of substances.
Examples of Lewis acids include hydrogen ion (H+), boron trifluoride (BF3), and aluminum chloride (AlCl3). Examples of Lewis bases include hydroxide ion (OH-), ammonia (NH3), and pyridine (C5H5N).
The Lewis theory is the most widely used theory of acids and bases because it is the most general and can explain the behavior of the widest range of substances.
Acid-Base Reactions, Acid-base theories worksheet answer key
Acid-base reactions are chemical reactions that involve the transfer of protons or electron pairs. These reactions are typically classified as neutralization reactions, in which an acid and a base react to form a salt and water, or as proton-transfer reactions, in which an acid donates a proton to a base.
Examples of acid-base reactions include the reaction of hydrochloric acid with sodium hydroxide to form sodium chloride and water, and the reaction of acetic acid with ammonia to form ammonium acetate.
The strength of an acid or base is determined by its ability to donate or accept protons or electron pairs. Strong acids and bases donate or accept protons or electron pairs easily, while weak acids and bases donate or accept protons or electron pairs less easily.
FAQ Section
What is the Arrhenius definition of an acid?
An Arrhenius acid is a substance that produces hydrogen ions (H+) when dissolved in water.
What are the advantages of the Lewis theory over the Arrhenius and Brønsted-Lowry theories?
The Lewis theory is more general and can be applied to a wider range of reactions, including those that do not involve the transfer of protons.
What is the relationship between pH and pOH?
pH + pOH = 14